
ELECTRONIC NORMALIZER ("EN")Autonomous Electrostimulator of Gastrointestinal Tract And MucosaTechnical conditions 9444-014-11555014-97 scetch MASI 943115.001 author, patentee and manufacturer - RPA "Ecomed", Russia, Moscow |
Appendix to survey of clinical test of AES - "EN" |
Comparative results
The highest efficiency in reduction of the most atherogenic fractions of cholesterol of lipoproteins of low density!
% of initial value alteration
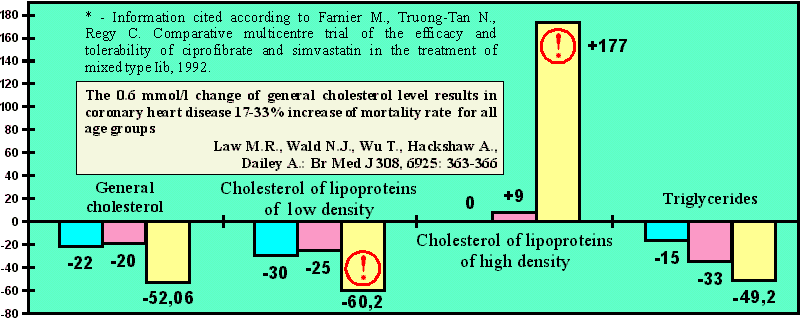
| Simvastatin* "ZOCOR" (effect in 8 weeks, 10 mg a day) | ||
| Ciprofibrate* "LIPANOR" (effect in 8 weeks, 100 mg a day) | ||
| "EN" (2 capsules in 30 days, effect in 1 week after reception of the 1st capsule) patent ¹ 2071368, international patent ¹ PCT/RU96/00023 |
Additional effects in comparison with Ciprofibrate
| Ciprofibrate (white tablets shaping in capsule form with raised impression Lipanor on one side demarcation strip on the other one) |
 |
(metallic capsule with laser marking  Electronic normalizator NPO "Ecomed" patent 207136 on the insulator between two caps, personal number and little star on capsule's caps Electronic normalizator NPO "Ecomed" patent 207136 on the insulator between two caps, personal number and little star on capsule's caps
|
pregnancy, kidneys and liver diseases
|
 |
mechanical intestinal obstruction
|